Stereoselective synthesis of the viridiofungin analogue NA808 from a chiral tetrahydrofuran-carboxylic acid†
Abstract
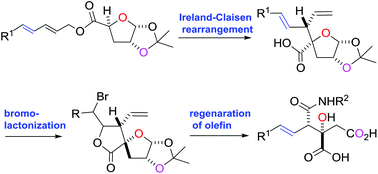
The viridiofungin analogue NA808 was synthesized by the stereoselective Ireland–Claisen rearrangement of dienylmethyl ester, regioselective bromolactonization of β-divinylpropanoic acid and retro-bromolactonization.


 Please wait while we load your content...
Please wait while we load your content...