Total synthesis of (+)-herboxidiene/GEX 1A†
Abstract
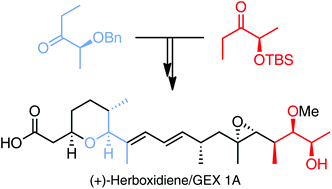
A total synthesis of (+)-herboxidiene/GEX 1A has been accomplished from (R)- and (S)-lactate esters in a highly efficient manner. Key steps of the synthesis involve substrate-controlled titanium-mediated aldol reactions from chiral lactate-derived ethyl ketones, an oxa-Michael cyclization, an Ireland–Claisen rearrangement, and a Suzuki coupling. Furthermore, computational studies of the oxa-Michael reaction have unveiled the dramatic influence of intramolecular hydrogen bonds on the stereochemical outcome of such cyclizations, whereas biological analyses have clearly proved the important cytoxicity of (+)-herboxidiene/GEX 1A.


 Please wait while we load your content...
Please wait while we load your content...