First asymmetric total synthesis of aspergillide D†
Abstract
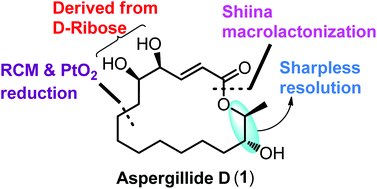
The first asymmetric total synthesis of a 16-membered macrolide, aspergillide D, is described. The chiral centers of the acid are derived from D-ribose and the alcohol subunit from 1,8-octane diol through Sharpless kinetic resolution, respectively. The other key reactions include Yamaguchi esterification, ring-closing metathesis reaction, and Shiina macrolactonization to construct the fully functionalized macrocycle.


 Please wait while we load your content...
Please wait while we load your content...