Modulation of redox potentials utilizing the flexible coordination sphere of a penta-coordinate complex in the solid state†
Abstract
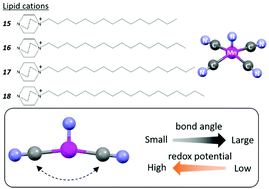
Slight changes in the coordination structure of the manganese(V)-nitrido anionic complex, [MnV(N)(CN)4]2−, induced by using a “lipid package” approach markedly made an impact on the corresponding redox potentials. The single crystals of four lipid assemblies, [dabco-(CH2)n−1-CH3]2[Mn(N)(CN)4(H2O)]·xH2O (n = 15, 16, 17 and 18; dabco = 1,4-diazabicyclo[2,2,2]octane), were synthesized and solid-state cyclic voltammetric studies demonstrated that the [MnV(N)(CN)4]2− anions with smaller “cross” NC–Mn–CN bond angles exhibit higher redox potentials. The observed trend reflects the energy change associated with the structural transformation from [MnV(N)(CN)4]2− to [MnVI(N)(CN)4]2− and is supported by the results of DFT calculations. The NC–Mn–CN bond angles in the flexible [Mn(N)(CN)4]2− structure exhibit excellent correlation with the redox potentials of the complexes in the solid state.


 Please wait while we load your content...
Please wait while we load your content...