Cu2+ recognition by N,N′-benzylated bis(amino amides)†
Abstract
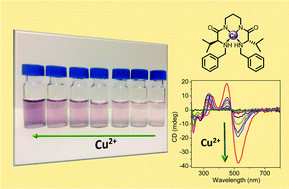
Two new C2-symmetric N,N′-benzylated bis(amino amides) have been synthesised and their interaction with different transition metals studied using a variety of techniques including UV-Vis and CD spectroscopy or ESI-MS. The determination of the corresponding stability constants with Cu2+ has been possible, in H2O/CH3CN 7/3 v/v, for one of these ligands (4) using potentiometric titrations. The results obtained reveal that N-benzylation affords significant changes to their properties and is accompanied by an appreciable decrease in the corresponding complexation stability constants. However, this, along with the low kinetics associated to Ni2+, facilitates the recognition of Cu2+ by 4 that can be followed by the naked-eye up to the submillimolar range. Very interestingly, the chiral nature of this ligand provides an intense and well defined CD curve for the corresponding Cu2+ complex, very sensitive to the coordination geometry, facilitating the analysis of this interaction even at the μM range. The formation by both ligands (3 and 4) of square planar complexes with Cu2+ and Ni2+ displaying a 1 : 1 stoichiometry was confirmed by their X-ray crystal structures.


 Please wait while we load your content...
Please wait while we load your content...