Borylated oximes: versatile building blocks for organic synthesis†
Abstract
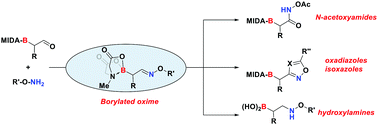
Herein, we demonstrate the synthesis and functionalization of α-boryl aldoximes from α-boryl aldehydes, with no sign of C-to-N boryl migration. Selective modification of the oxime functionality enables access to a wide range of borylated compounds, such as borylated heterocycles and N-acetoxyamides. By reducing the α-boryl aldoximes, MIDA deprotection yields the corresponding β-boryl hydroxylamines. As part of this study, we also demonstrate the utility of the boryl aldoxime motif in peptide conjugation.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...