Magnetically separable Fe3O4@chitin as an eco-friendly nanocatalyst with high efficiency for green synthesis of 5-substituted-1H-tetrazoles under solvent-free conditions†
Abstract
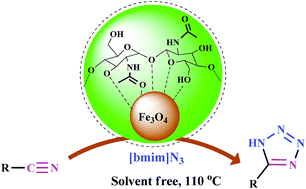
The present study describes an efficient, eco-friendly and simple method for the synthesis of 5-substituted-1H-tetrazoles catalyzed by magnetite–chitin (Fe3O4@chitin) as a green and recyclable catalyst. Fe3O4@chitin was initially prepared using hydrothermal synthesis. Subsequently, the structure, morphology, and magnetic properties of the prepared nanocatalyst were studied with some different spectroscopic, microscopic and thermogravimetric techniques such as FT-IR, XRD, SEM, TEM, VSM and TGA. Obtained results showed that Fe3O4@chitin nanoparticles exhibited uniform cubic shape and were well monodispersed. Also, magnetic measurement revealed that the synthesized nanocatalyst had superparamagnetic features. The application of this new nanocatalyst allows the synthesis of a variety of tetrazoles through the reaction of nitriles with 1-butyl-3-methylimidazolium azide ([bmim][N3]) under solvent-free conditions. This synthetic pathway is a green protocol offering significant advantages, such as excellent yield of products in short reaction times, mild reaction conditions, minimization of chemical waste, easy preparation of the catalyst and its recyclability up to six cycles without any considerable loss of efficiency.

 Please wait while we load your content...
Please wait while we load your content...