Substitution dependent stereoselective construction of bicyclic lactones and its application to the total synthesis of pyranopyran, tetraketide and polyrhacitide A†
Abstract
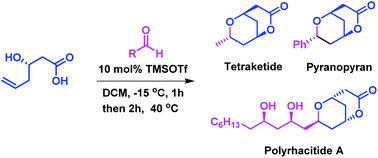
A novel bicyclization strategy has been developed for the stereoselective synthesis of bicyclic lactones, i.e. 7-aryl or alkyl-2,6-dioxabicyclo[3.3.1]nonan-3-ones through a domino cyclization of (R)-3-hydroxyhex-5-enoic acid with an aldehyde in the presence of 10 mol% trimethylsilyltriflate under mild conditions. The salient features of this methodology are high yields, excellent selectivity, low catalyst loading and faster reaction times. This method has been successfully applied to the total synthesis of pyranopyran, tetraketide and polyrhacitide A.

 Please wait while we load your content...
Please wait while we load your content...