Total synthesis of mangiferin, homomangiferin, and neomangiferin†
Abstract
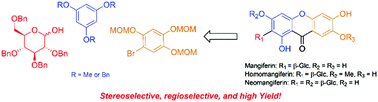
Total synthesis of mangiferin, homomangiferin, and neomangiferin, three C-glycosyl xanthone natural products with a wide spectrum of pharmacological effects, has been achieved starting from 2,3,4,6-tetra-O-benzyl-α/β-D-glucopyranose. The key steps involve a stereoselective Lewis acid promoted C-glycosylation of protected phloroglucinol with tetrabenzylglucopyranosyl acetate and a highly regioselective base-induced cyclization for the construction of the core xanthone skeleton.

 Please wait while we load your content...
Please wait while we load your content...