Synthesis of substituted benzooxaborinin-1-ols via palladium-catalysed cyclisation of alkenyl- and alkynyl-boronic acids†
Abstract
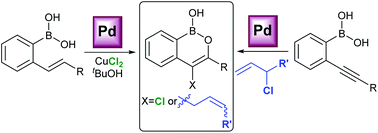
Two new palladium-catalysed reactions have been developed for the synthesis of stable 4-substituted benzooxaborinin-1-ols. A palladium-catalysed cyclisation of ortho-alkenylbenzene boronic acids can be used to access 4-chlorobenzooxaborinin-1-ols via a Wacker-type oxidation and chlorination. Alternatively, ortho-alkynylbenzene boronic acids undergo a palladium-catalysed oxyallylation reaction to provide 4-allylbenzooxaborinin-1-ols.


 Please wait while we load your content...
Please wait while we load your content...