Synthesis of substituted tetrahydroisoquinolines by lithiation then electrophilic quench†
Abstract
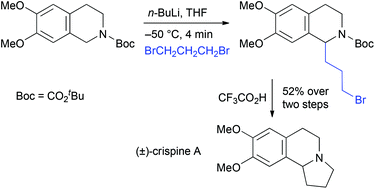
Substituted N-tert-butoxycarbonyl (Boc)-1,2,3,4-tetrahydroisoquinolines were prepared and treated with n-butyllithium in THF at −50 °C to test the scope of the metallation and electrophilic quench. The lithiation was optimised by using in situ ReactIR spectroscopy and the rate of rotation of the carbamate was determined. The 1-lithiated intermediates could be trapped with a variety of electrophiles to give good yields of 1-substituted tetrahydroisoquinoline products. Treatment with acid or reduction with LiAlH4 allows conversion to the N–H or N–Me compound. The chemistry was applied to the efficient total syntheses of the alkaloids (±)-crispine A and (±)-dysoxyline.
 Please wait while we load your content...
Please wait while we load your content...