Synthetic approaches towards alkaloids bearing α-tertiary amines
Abstract
Covering: up to August 2015
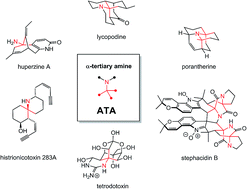
Alkaloids account for some of the most beautiful and biologically active natural products. Although they are usually classified along biosynthetic criteria, they can also be categorized according to certain structural motifs. Amongst these, the α-tertiary amine (ATA), i.e. a tetrasubstituted carbon atom surrounded by three carbons and one nitrogen, is particularly interesting. A limited number of methods have been described to access this functional group and fewer still are commonly used in synthesis. Herein, we review some approaches to asymmetrically access ATAs and provide an overview of alkaloid total syntheses where those have been employed.

 Please wait while we load your content...
Please wait while we load your content...