Comprehensive mapping of O-GlcNAc modification sites using a chemically cleavable tag†
Abstract
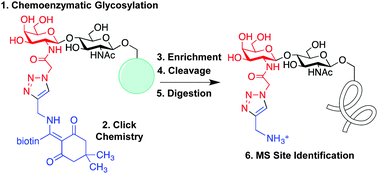
The post-translational modification of serine or threonine residues of proteins with a single N-acetylglucosamine monosaccharide (O-GlcNAcylation) is essential for cell survival and function. However, relatively few O-GlcNAc modification sites have been mapped due to the difficulty of enriching and detecting O-GlcNAcylated peptides from complex samples. Here we describe an improved approach to quantitatively label and enrich O-GlcNAcylated proteins for site identification. Chemoenzymatic labelling followed by copper(I)-catalysed azide–alkyne cycloaddition (CuAAC) installs a new mass spectrometry (MS)-compatible linker designed for facile purification of O-GlcNAcylated proteins from cell lysates. The linker also allows subsequent quantitative release of O-GlcNAcylated proteins for downstream MS analysis. We validate the approach by unambiguously identifying several established O-GlcNAc sites on the proteins α-crystallin and O-GlcNAc transferase (OGT), as well as discovering new, previously unreported sites on OGT. Notably, these novel sites on OGT lie in key functional domains of the protein, underscoring how this site identification method may reveal important biological insights into protein activity and regulation.
- This article is part of the themed collection: Protein Labelling

 Please wait while we load your content...
Please wait while we load your content...