Development of silica-supported frustrated Lewis pairs: highly active transition metal-free catalysts for the Z-selective reduction of alkynes†
Abstract
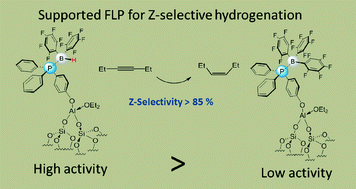
Supported Lewis acid/base systems based on a triphenyl phosphine fragment and Piers' reagent (HB(C6F5)2) or BArF have been prepared and characterized. Both materials show unprecedented catalytic activity in the Z-selective hydrogenation of 3-hexyne to Z-3-hexene with a selectivity up to 87%. Other alkynes can also be hydrogenated Z-selectively, albeit with moderate yields. The activity of the supported phosphine/HB(C6F5)2 adduct is similar to the only homogeneous example reported thus far based on bridged B/N frustrated Lewis pairs under high hydrogen pressure. Importantly, this transition metal-free supported catalyst was recycled five times in the challenging selective hydrogenation of a non-polar unactivated alkyne.

 Please wait while we load your content...
Please wait while we load your content...