Defects in the calcium-binding region drastically affect the cadherin-like domains of RET tyrosine kinase†
Abstract
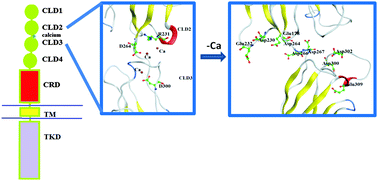
Mutations in the rearranged during transfection (RET) tyrosine kinase gene leading to gain or loss of function have been associated with the development of several human cancers and Hirschsprung's disease (HSCR). However, to what extent these mutations affect individual bio-molecular functions remains unclear. In this article, the functionally significant mutations in the RET CLD1–4 calcium-binding site which lead to HSCR, and depletion of calcium ions in the RET CLD1–4 calcium binding site, were investigated by molecular dynamics simulations – to understand the mechanistic action of the mutations or loss of calcium ions in altering the protein kinase structure, dynamics, and stability. The mutations or loss of calcium ions change the local conformation and change the free energy landscape. Specifically, the mutations and loss of calcium ions decrease the radius of gyration of the whole structure, leading to improper protein folding and GFL–GFRα contact site reduction. Furthermore, based on the most populated conformation in the wildtype MD simulations, a pharmacophore was generated by fragment docking to identify key features of the possible inhibitors targeting the calcium binding site. Overall, the findings may provide useful structural insights into the molecular mechanism underlying RET calcium-binding site mutations and assist in development of novel drugs targeting the extracellular ligand contact site of wildtype RET.

 Please wait while we load your content...
Please wait while we load your content...