Rhodium-catalyzed transformation of heteroaryl aryl ethers into heteroaryl fluorides†
Abstract
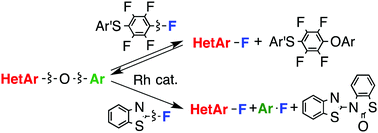
A rhodium complex catalyzed the conversion of the C–O bond of heteroaryl aryl ethers to the C–F bond. The reaction of (4-chlorophenylthio)pentafluorobenzene with heteroaryl aryl ethers provided heteroaryl fluorides and heteroaryl (4-chlorophenylthio)tetrafluorophenyl ethers; this involved the cleavage of a single heteroaryl C–O bond under equilibrium conditions. The reaction of heteroaryl aryl ethers with 2-fluorobenzothiazole in which two heteroaryl and aryl C–O bonds were cleaved provided heteroaryl fluorides and aryl fluorides. The reactions were applicable to five-membered and six-membered heteroaryl aryl ethers and also to diaryl ethers possessing one or two electron-withdrawing groups.

 Please wait while we load your content...
Please wait while we load your content...