On the existence of intramolecular one-electron Be–Be bonds†
Abstract
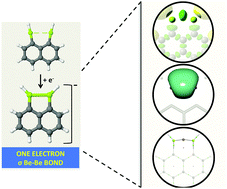
Although the Be–Be bond is extremely weak in Be2 dimers, we have shown that rather stable Be–Be one-electron sigma bonds are formed upon electron attachment to 1,8-diBeX-naphthalene derivatives. Wavefunction analyses corroborate the formation of Be–Be covalent linkages in which the extra electron is accommodated between the Be atoms as reflected in the dramatic shortening of the Be–Be distance with respect to the corresponding neutral molecule.

 Please wait while we load your content...
Please wait while we load your content...