Decarboxylative dearomatization and mono-α-arylation of ketones†
Abstract
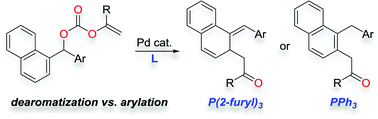
We report the first example of a palladium-catalyzed decarboxylative dearomatization reaction that occurs via Pd-π-benzyl intermediates. In fact, the Pd-catalyzed decarboxylative cross-coupling reaction of benzyl enol carbonates can lead to either the dearomatized alicyclic ketones or α-monoarylated ketone products depending on the catalyst and ligand employed.

 Please wait while we load your content...
Please wait while we load your content...