Asymmetric dual catalysis via fragmentation of a single rhodium precursor complex†
Abstract
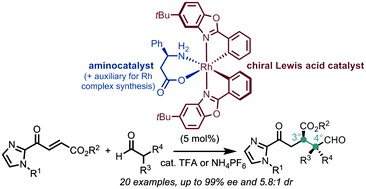
A strategy for dual transition metal catalysis and organocatalysis is reported via the in situ disintegration of a single rhodium complex. The hereby generated chiral Lewis acid and L-β-phenylalanine synergistically catalyze the Michael addition of α,α-disubstituted aldehydes to α,β-unsaturated 2-acyl imidazoles under the formation of vicinal quaternary/tertiary stereocenters. Conveniently, the chiral-at-metal rhodium catalyst can be synthesized in just two steps starting from rhodium trichloride without the need for any chromatography.

 Please wait while we load your content...
Please wait while we load your content...