Copper mediated decarboxylative direct C–H arylation of heteroarenes with benzoic acids†
Abstract
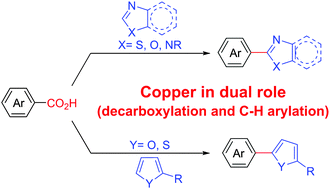
Decarboxylative coupling reactions to date require a stoichiometric oxidant (such as copper and silver salts) for decarboxylation purposes along with a metal catalyst (e.g. palladium) for cross-coupling. In this communication, an economic and sustainable approach by using a simple copper salt was developed in the presence of molecular oxygen as the sole oxidant. A wide range of 5-membered heteroarenes undergo aryl–heteroaryl cross-coupling with electron deficient aryl carboxylic acids.

 Please wait while we load your content...
Please wait while we load your content...