A highly convergent synthesis of the C1–C31 polyol domain of amphidinol 3 featuring a TST-RCM reaction: confirmation of the revised relative stereochemistry†
Abstract
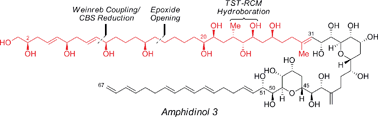
The concise enantioselective synthesis of the revised C1–C31 fragment of the polyketide amphidinol 3 was accomplished in 16 steps and 12.8% overall yield. Salient features of the strategy include chemoselective Weinreb amide coupling and concomitant CBS reduction for the preparation of the C1–C15 tris-syn-1,5-diol motif and a temporary silicon-tethered ring-closing metathesis (TST-RCM) reaction in combination with a diastereoselective hydroboration for the construction of the C16–C31 polypropionate fragment. The union of the fragments was accomplished by a regioselective ring-opening of the terminal epoxide with a phenyl sulfone stabilized carbanion, which upon reduction and deprotection permits a comparison of the relative configuration with the natural product.

 Please wait while we load your content...
Please wait while we load your content...