In vitro and in vivo comparative and competitive activity-based protein profiling of GH29 α-l-fucosidases†
Abstract
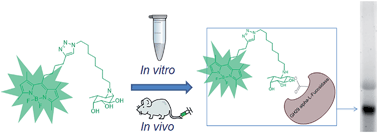
GH29 α-L-fucosidases catalyze the hydrolysis of α-L-fucosidic linkages. Deficiency in human lysosomal α-L-fucosidase (FUCA1) leads to the recessively inherited disorder, fucosidosis. Herein we describe the development of fucopyranose-configured cyclophellitol aziridines as activity-based probes (ABPs) for selective in vitro and in vivo labeling of GH29 α-L-fucosidases from bacteria, mice and man. Crystallographic analysis on bacterial α-L-fucosidase confirms that the ABPs act by covalent modification of the active site nucleophile. Competitive activity-based protein profiling identified L-fuconojirimycin as the single GH29 α-L-fucosidase inhibitor from eight configurational isomers.

 Please wait while we load your content...
Please wait while we load your content...