Palladium-catalyzed cross-coupling of α-bromocarbonyls and allylic alcohols for the synthesis of α-aryl dicarbonyl compounds†
Abstract
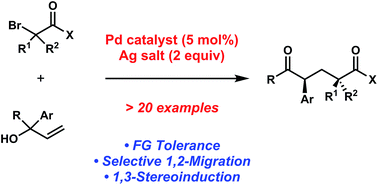
The palladium-catalyzed coupling of olefins and organohalides is a versatile approach for synthesizing complex molecules from simple starting materials. We have developed a palladium-catalyzed coupling of α-bromocarbonyl compounds with allylic alcohols for the generation of acyclic aryl-substituted dicarbonyl compounds. The reaction proceeds via a tandem olefin insertion of an α-acyl radical followed by a 1,2-aryl migration. In addition to providing preliminary evidence for a free radical mediated mechanism, we demonstrate unprecedented levels of 1,3-stereoinduction for the 1,2-migration step.

 Please wait while we load your content...
Please wait while we load your content...