In(OTf)3-catalyzed synthesis of 2-styryl quinolines: scope and limitations of metal Lewis acids for tandem Friedländer annulation–Knoevenagel condensation†
Abstract
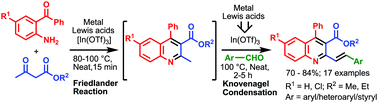
The catalytic potential of different metal Lewis acids has been assessed for the one-pot tandem Friedländer annulation and Knoevenagel condensation involving 2-aminobenzophenone, ethyl acetoacetate, and benzaldehyde to form 2-styryl quinoline under solvent free conditions. While various metal Lewis acids were effective in promoting the Friedländer annulation step, In(OTf)3 was the only effective catalyst for the subsequent Knoevenagel condensation reaction suggesting In(OTf)3 as the stand-alone catalyst for the tandem Friedländer–Knoevenagel reaction to form 2-styryl quinolines. The protocol is compatible with different variations of aromatic/hetero-aromatic aldehydes and α,β unsaturated aromatic aldehydes giving highly functionalized 2-aryl/heteroaryl vinyl quinolines. The catalyst can be recovered and reused to afford the desired product in very good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...