Sulfate recognition by a hexaaza cryptand receptor†
Abstract
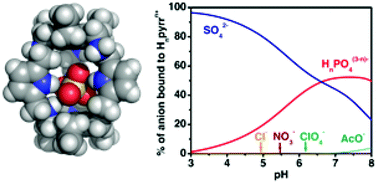
A hexamine macrobicycle with pyrrolyl spacers was evaluated as an anion receptor in its protonated forms. The protonation constants of the receptor, as well as its association constants with Cl−, NO3−, AcO−, ClO4−, H2PO4−, and SO42− were determined by potentiometry at 298.2 ± 0.1 K in H2O–MeOH (50 : 50 v/v) and at an ionic strength of 0.10 ± 0.01 M in KTsO. These studies revealed that the Hnpyrrn+ receptor has a very high effective association constant value for the SO42− at pH 4.0 (log Keff = 6.42), and it is selective for the uptake of this anion in the presence of the other studied anionic substrates. In particular, the receptor showed very high SO42−/NO3− selectivity. Using the indicator-displacement approach the receptor is able to signal the presence of sulfate by a change of color. Single crystal X-ray diffraction determination of [(H6pyrr)(SO4)(H2O)3](SO4)2·9.3H2O revealed the presence of one sulfate anion inside the receptor cavity and showed that the encapsulation of the anion is favored by an array of nine hydrogen bonding interactions, including N–H⋯O, C–H⋯O and water-mediated ones.
 Please wait while we load your content...
Please wait while we load your content...