Ring-opening polymerization of lactide using chiral salen aluminum complexes as initiators: high productivity and stereoselectivity†
Abstract
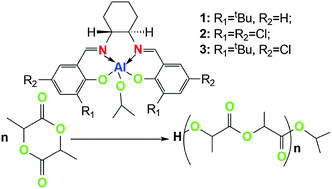
A family of aluminum complexes bearing chiral salen ligands derived from (R,R)-1,2-diammoniumcyclohexane mono-(+)-tartrate salt and modified by salicylaldehyde were prepared. The complexes were characterized by 1H, 13C NMR and elemental analysis. These complexes were used as initiators for the ring-opening polymerization (ROP) of L-lactide and rac-lactide. Complex 2 (R1 = R2 = Cl) showed the highest activity among these complexes for the ROP of L-lactide, and complex 3 (R1 = tBu, R2 = Cl) showed the highest stereoselectivity for the ROP of rac-lactide with enriched isotactic polylactide with a Pm of 0.91. The kinetic data of the polymerization employing complex 3 as initiator indicated that the polymeric rate was first-order in both lactide and in initiator.

 Please wait while we load your content...
Please wait while we load your content...