A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass†
Abstract
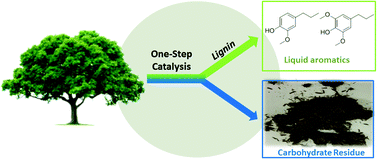
Current biomass utilization processes do not make use of lignin beyond its heat value. Here we report on a bimetallic Zn/Pd/C catalyst that converts lignin in intact lignocellulosic biomass directly into two methoxyphenol products, leaving behind the carbohydrates as a solid residue. Genetically modified poplar enhanced in syringyl (S) monomer content yields only a single product, dihydroeugenol. Lignin-derived methoxyphenols can be deoxygenated further to propylcyclohexane. The leftover carbohydrate residue is hydrolyzed by cellulases to give glucose in 95% yield, which is comparable to lignin-free cellulose (solka floc). New conversion pathways to useful fuels and chemicals are proposed based on the efficient conversion of lignin into intact hydrocarbons.
- This article is part of the themed collection: 2015 most accessed Green Chemistry articles

 Please wait while we load your content...
Please wait while we load your content...