When can ionic liquids be considered readily biodegradable? Biodegradation pathways of pyridinium, pyrrolidinium and ammonium-based ionic liquids†
Abstract
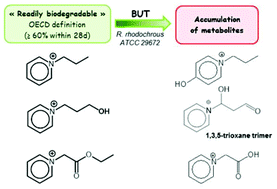
Ionic liquids have been the subject of intense interest over the past decade due to their unique structures which can be tuned to modify the physicochemical properties of the solvent. Nevertheless, industrial processes rarely involve ILs, partly because our understanding of their environmental impact and biodegradability is still in its infancy. The biodegradability criteria for chemical compounds have been defined by the OECD, according to standard protocols in which a chemical is exposed to microbes in an activated sludge over a period of 28 days. However, most reports on IL biodegradation have concentrated on ultimate biodegradability, and have neglected to identify the metabolites along the biodegradative pathway. In fact, intermediate metabolites can be more toxic than the parent molecule and may have a completely different environmental profile. We have studied the antimicrobial activities and biodegradation kinetics of ten pyridinium, pyrrolidinium and ammonium-based ionic liquids incubated with either a pure strain of Rhodococcus rhodochrous ATCC 29672 or an activated sludge, and have also determined their degradation pathways using 1H NMR and LC-MS, with accompanying control experiments under abiotic conditions. Several intermediary metabolites were identified and quantified. All the ILs (except a long-chain alkylpyridinium in C6) proved to be “readily biodegradable” with the pure strain (80–100% degradation) under the conditions of the test, which was not the case with the activated sludges; however, in 3 cases of 10, the biodegradation resulted in an undesirable accumulation of metabolites.

 Please wait while we load your content...
Please wait while we load your content...