Coinage metal complexes supported by a “PN3P” scaffold†
Abstract
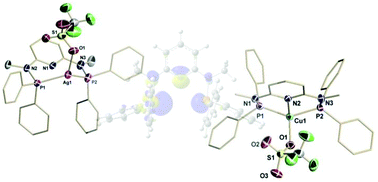
A series of monovalent group 11 complexes, [2,6-{Ph2PNMe}2(NC5H3)]CuBr 1, [2,6-{Ph2PNMe}2(NC5H3)]CuOTf 2, [2,6-{Ph2PNMe}2(NC5H3)]AgOTf 3, and [2,6-{Ph2PNMe}2(NC5H3)](AuCl)24, supported by a neutral PN3P ligand have been synthesized and characterized by multinuclear NMR and single crystal X-ray diffraction studies. The variation of the coordination properties were analyzed and electronic structure calculations have been carried out to provide insight on the bonding details in these complexes. The Cu(I) complexes displayed an unusual coordination geometry with a tridentate pincer ligand and an overall four coordinate trigonal pyramidal geometry. In contrast the Ag(I) analogue displayed a bidentate κ2-P,P′ ligation leaving the pyridyl-N atom uncoordinated and yielding a pyramidalized trigonal planar geometry around Ag. The bimetallic Au(I) complex completed the series and displayed a monodentate P-bonded ligand and a linear coordination geometry.

 Please wait while we load your content...
Please wait while we load your content...