Complexes of (η6-benzene)ruthenium(ii) with 1,4-bis(phenylthio/seleno-methyl)-1,2,3-triazoles: synthesis, structure and applications in catalytic activation of oxidation and transfer hydrogenation†
Abstract
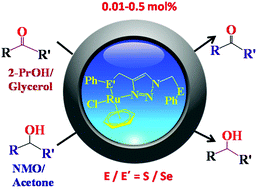
1,4-Bis(phenylthio/seleno methyl)-1,2,3-triazoles (L1–L4) synthesized by a ‘Click’ reaction react with [{(η6-C6H6)RuCl(μ-Cl)}]2 and NH4PF6 resulting in complexes [(η6-C6H6)RuClL]PF6 (1–4 for L = L1–L4) in which the ligands coordinate in a bidentate mode through S/Se and N of triazole. The CH2EPh (E = S or Se) attached to nitrogen of triazole remains pendent. Ligands and complexes have been authenticated with multinuclei NMR, IR and HR-MS. Single crystal structures of complexes 1–4 have been solved. The Ru–S and Ru–Se bond lengths (Å) are respectively 2.388(2)/2.3902(19) and 2.5007(4)/2.5262(19). The disposition of benzene ring, N, S/Se and Cl around Ru is of a piano stool type. For catalytic oxidation of alcohols [Oppenauer-type and with N-methylmorpholine-N-oxide (NMO)] and transfer hydrogenation (TH) of carbonyl compounds [with 2-propanol and glycerol] all the four complexes have been found efficient. The optimum catalyst loadings (in mol%) are: 0.01 (NMO), 0.1 (Oppenauer), 0.01 (TH with 2-propanol) and 0.5 (TH with glycerol). Interestingly, time profiles (under optimum conditions) of two catalytic oxidations and TH's are almost similar, suggesting that they are competitive on appropriate catalyst loading. DFT calculations are consistent with somewhat low reactivity of 1 in comparison to those of 2–4.

 Please wait while we load your content...
Please wait while we load your content...