Exhibition of the Brønsted acid–base character of a Schiff base in palladium(ii) complex formation: lithium complexation, fluxional properties and catalysis of Suzuki reactions in water†
Abstract
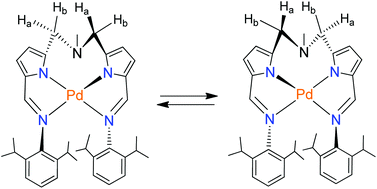
The reaction of the dialdehyde N,N-di(α-formylpyrrolyl-α-methyl)-N-methylamine 1 with two equiv. of 2,6-diisopropylaniline yielded two Schiff bases: bis(iminopyrrolylmethyl)amine (H2L) and its hydrolyzed monoimino compound (H2L′) after column separation. The dimeric lithium complex [(HL)Li]2 (2) containing the monoanionic form of H2L was obtained by treating H2L with nBuLi. The presence of both proton donors and acceptors causes the diimino compound H2L to undergo tautomerization to exhibit an amine–azafulvene structure, though the central amine nitrogen competes for a proton. As a result, in the presence of Pd2+ ions, the cationic complex [Pd(Cl)(H2L)][Cl] (3) containing one pendant amine–azafulvene arm and the protonated central amine nitrogen was obtained. Its X-ray structure showed that the bond distances are reversed for the imino-pyrrole moiety relative to those in the structure of H2L. However, the reaction of H2L with [Pd(OAc)2] afforded the neutral complex [PdL] (4) containing the dianionic form of the ligand. The reaction of H2L′ with [PdCl2(PhCN)2] yielded a zwitterionic complex [PdCl2(H2L′)] (5) owing to the presence of the central amine nitrogen. The formation of these palladium complexes with the features mentioned above can be explained by invoking the Brønsted acid–base character of the Schiff base. Complex 4 is fluxional owing to the up and down movements of the palladium square plane formed by two 5-membered palladacycles, which causes the interconversion of its enantiomers and is studied by the variable temperature 1H NMR method. Furthermore, both complexes 3 and 4 are precatalysts for the Suzuki–Miyaura cross-coupling reaction in water. Sterically encumbered and electronically different substrates including activated aryl chlorides and benzyl halides gave the coupled products in very good yields. The reaction proceeds even at room temperature and in the presence of a large excess amount of mercury.

 Please wait while we load your content...
Please wait while we load your content...