New palladium(ii) and platinum(ii) 5,5-diethylbarbiturate complexes with 2-phenylpyridine, 2,2′-bipyridine and 2,2′-dipyridylamine: synthesis, structures, DNA binding, molecular docking, cellular uptake, antioxidant activity and cytotoxicity†
Abstract
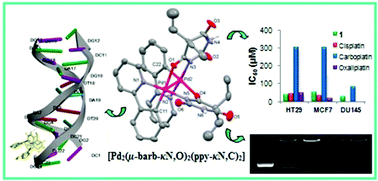
Novel palladium(II) and platinum(II) complexes of 5,5-diethylbarbiturate (barb) with 2-phenylpyridine (Hppy), 2,2′-bipyridine (bpy) and 2,2′-dipyridylamine (dpya) have been prepared and characterized by elemental analysis, IR, UV-Vis, NMR and ESI-MS. Single-crystal diffraction measurements show that complex 1 consists of binuclear [Pd2(μ-barb-κN,O)2(ppy-κN,C)2] moieties, while complexes 3–5 are mononuclear, [M(barb-κN)2(L-κN,N′)] (L = bpy or dpya). 6 has a composition of [Pt(dpya-κN,N′)2][Ag(barb-κN)2]2·4H2O and 2 was assumed to have a structure of [Pt(barb-κN)(Hppy-κN)(ppy-κN,C)]·3H2O. The complexes were found to exhibit significant DNA binding affinity by a non-covalent binding mode, in accordance with molecular docking studies. In addition, complexes 1 and 2 displayed strong binding with supercoiled pUC19 plasmid DNA. Cellular uptake studies were performed to assess the subcellular localization of the selected complexes. A moderate radical scavenging activity of 1 and 2 was confirmed by DPPH and ABTS tests. Complexes 1, 2, and 5 showed selectivity against HT-29 (colon) cell line.

 Please wait while we load your content...
Please wait while we load your content...