Regioselective aerobic oxidative Heck reactions with electronically unbiased alkenes: efficient access to α-alkyl vinylarenes†
Abstract
Branched-selective oxidative Heck coupling reactions have been developed between arylboronic acids and electronically unbiased terminal alkenes. The reactions exhibit high catalyst-controlled regioselectivity favoring the less common branched isomer. The reactions employ a catalyst composed of Pd(TFA)2/dmphen (TFA = trifluoroacetate, dmphen = 2,9-dimethyl-1,10-phenanthroline) and proceed efficiently at 45–60 °C under 1 atm of O2 without requiring other additives. A broad array of functional groups, including aryl halide, allyl silane and carboxylic acids are tolerated.
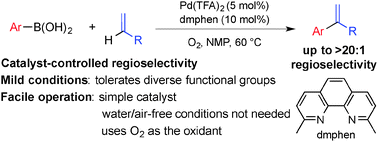

 Please wait while we load your content...
Please wait while we load your content...