Synthesis of oxazolidine-2,4-diones by a tandem phosphorus-mediated carboxylative condensation–cyclization reaction using atmospheric carbon dioxide†
Abstract
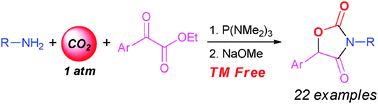
The oxazolidine-2,4-dione motif is found frequently in biologically important compounds. A tandem phosphorus-mediated carboxylative condensation of primary amines and α-ketoesters/base-catalyzed cyclization reaction have been developed. These processes provide a novel and convenient access to various oxazolidine-2,4-diones in a one-pot fashion using atmospheric carbon dioxide and readily available substrates under very mild and transition-metal-free conditions.

 Please wait while we load your content...
Please wait while we load your content...