Amine α-heteroarylation via photoredox catalysis: a homolytic aromatic substitution pathway†
Abstract
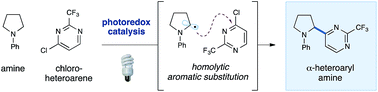
The direct α-heteroarylation of tertiary amines has been accomplished via photoredox catalysis to generate valuable benzylic amine pharmacophores. A variety of five- and six-membered chloroheteroarenes are shown to function as viable coupling partners for the α-arylation of a diverse range of cyclic and acyclic amines. Evidence is provided for a homolytic aromatic substitution mechanism, in which a catalytically-generated α-amino radical undergoes direct addition to an electrophilic chloroarene.

 Please wait while we load your content...
Please wait while we load your content...