A solvent-resistant halogen bond†
Abstract
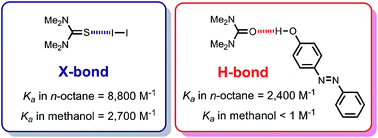
The effect of solvent on the stabilities of complexes involving a single H-bond or halogen-bond (X-bond) has been quantified. Association constants for binary complexes of 4-(phenylazo)phenol, molecular iodine, tetramethylurea and tetramethylthiourea have been measured in fifteen different solvents by UV/vis absorption and 1H NMR titration experiments. The stabilities of the H-bonded complexes decrease by more than three orders of magnitude with increasing solvent polarity. In contrast, the X-bonded complex of molecular iodine with tetramethylthiourea is remarkably insensitive to the nature of the solvent (association constants measured in alkanes and alcohols are similar). The results suggest that, in contrast to H-bonds, where electrostatics determine thermodynamic stability, charge-transfer interactions make a major contribution to the stability of these X-bonded complexes rendering them resistant to increases in solvent polarity.

 Please wait while we load your content...
Please wait while we load your content...