Organocatalytic enantioselective α-amination of 5-substituted rhodanines: an efficient approach to chiral N,S-acetals†
Abstract
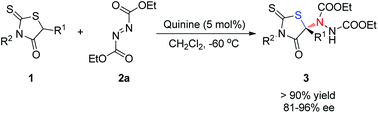
We report a highly efficient approach to constructing chiral N,S-acetals using 5-substituted rhodanines as sulfur-bound pronucleophiles catalyzed by natural cinchona alkaloids quinine or quinidine. This α-amination reaction has a broad substrate scope, and the products featuring both rhodanine and N,S-acetal structural motifs were obtained in high yields and excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...