Organocatalytic Michael addition–lactonisation of carboxylic acids using α,β-unsaturated trichloromethyl ketones as α,β-unsaturated ester equivalents†
Abstract
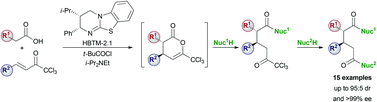
Isothiourea HBTM-2.1 catalyses the Michael addition–lactonisation of 2-aryl and 2-alkenylacetic acids and α,β-unsaturated trichloromethyl ketones. Ring-opening of the resulting dihydropyranones and subsequent alcoholysis of the CCl3 ketone with an excess of methanol gives a range of diesters in high diastereo- and enantioselectivity (up to 95 : 5 dr and >99% ee). Sequential addition of two different nucleophiles to a dihydropyranone gives the corresponding differentially substituted diacid derivative.

 Please wait while we load your content...
Please wait while we load your content...