Synthesis of oligonucleotides containing N,N-disubstituted 3-deazacytosine nucleobases by post-elongation modification and their triplex-forming ability with double-stranded DNA†
Abstract
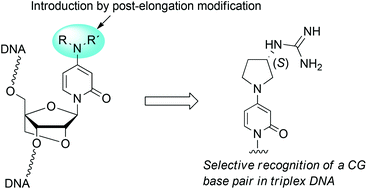
A phosphoramidite of a 2′-O,4′-C-methylene-bridged nucleoside, bearing 4-(2,4,6-triisopropylbenzenesulfonyloxy)pyridin-2-one as a nucleobase precursor, was synthesized and introduced into an oligonucleotide. Treatment with various secondary amines after elongating the oligonucleotide on an automated DNA synthesizer enabled facile and mild conversion of the precursor into the corresponding N,N-disubstituted 3-deazacytosine nucleobases. The evaluation of the triplex-forming ability of the synthesized oligonucleotides with double-stranded DNA showed that the nucleobase possessing the (3S)-3-guanidinopyrrolidine moiety can recognize a CG base pair with high sequence-selectivity and binding-affinity.

 Please wait while we load your content...
Please wait while we load your content...