Co(ii), Ni(ii) and Cu(ii) complexes with phenylthiazole and thiosemicarbazone-derived ligands: synthesis, structure and cytotoxic effects†
Abstract
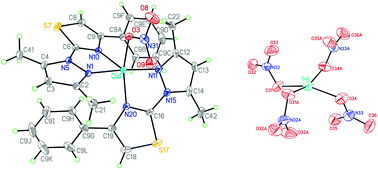
Complexes of Co(II), Ni(II) and Cu(II) with 2-(3,5-dimethyl-1H-pyrazole-1-yl)-4-phenyl-1,3-thiazole (a) and 3,5-dimethyl-1H-pyrazole-1-carbothioamide (b) ligands were synthesized. The crystal and molecular structures of four of them were determined by the X-ray diffraction method. In the complexes with ligand a the metal atom was bound via two nitrogen atoms, whereas ligand b interacted through the nitrogen and sulfur atoms. Comparing the coordination modes observed for both studied ligands, the thiocarbamoyl sulfur atom can participate in metal binding and in bridge formation, whereas the thiazole (aromatic) sulfur atom is not involved in the coordination. This effect seems to enhance (at least partially) conformational flexibility. Hydrogen bonds played a principal role in the crystal network formation of the analyzed compounds. The cytotoxic activity of the complexes against HL-60 and NALM-6 leukemia cells and the WM-115 melanoma cell line was also measured. The copper(II) complex with the thiosemicarbazone ligand (7b) exhibited relatively high cytotoxicity towards all tested tumor cells. The cytotoxicity of copper(II) complexes 7b and 9b against the melanoma WM-115 cells was over three times higher than that of cisplatin.

 Please wait while we load your content...
Please wait while we load your content...