Synthesis of a multifunctional alkoxysiloxane oligomer†
Abstract
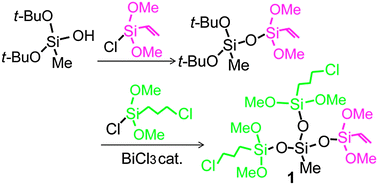
An alkoxysiloxane oligomer (1, SiMe[OSi(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(OMe)2][OSi(CH2)3Cl(OMe)2]2), containing vinyl and chloropropyl groups, was synthesized as a precursor for sol–gel synthesis. Di-tert-butoxymethylhydroxysilane (t-BuO)2MeSiOH was reacted with (MeO)2(CH2
CH2)(OMe)2][OSi(CH2)3Cl(OMe)2]2), containing vinyl and chloropropyl groups, was synthesized as a precursor for sol–gel synthesis. Di-tert-butoxymethylhydroxysilane (t-BuO)2MeSiOH was reacted with (MeO)2(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)SiCl to form (t-BuO)2MeSiOSi(CH
CH)SiCl to form (t-BuO)2MeSiOSi(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(OMe)2 which was further alkoxysilylated with Cl(CH2)3SiCl(OMe)2 to form 1. The 1H, 13C, 29Si NMR and HR-MS data confirmed the formation of 1, indicating the successful synthesis of an alkoxysiloxane oligomer possessing different kinds of functional groups by a chemoselective route. Hydrolysis of 1 under acidic conditions was completed in a few hours. The solution state 29Si NMR spectra of samples hydrolyzed and condensed at various reaction times show no signals due to species generated by the cleavage of the siloxane bonds in 1, indicating the validity of the synthesized substance as a precursor for the formation of hybrids with homogeneously distributed functional groups. Intramolecular condensation of 1 to form cyclic trisiloxane units proceeds more preferentially than intermolecular condensation.
CH2)(OMe)2 which was further alkoxysilylated with Cl(CH2)3SiCl(OMe)2 to form 1. The 1H, 13C, 29Si NMR and HR-MS data confirmed the formation of 1, indicating the successful synthesis of an alkoxysiloxane oligomer possessing different kinds of functional groups by a chemoselective route. Hydrolysis of 1 under acidic conditions was completed in a few hours. The solution state 29Si NMR spectra of samples hydrolyzed and condensed at various reaction times show no signals due to species generated by the cleavage of the siloxane bonds in 1, indicating the validity of the synthesized substance as a precursor for the formation of hybrids with homogeneously distributed functional groups. Intramolecular condensation of 1 to form cyclic trisiloxane units proceeds more preferentially than intermolecular condensation.

 Please wait while we load your content...
Please wait while we load your content...