Semi-catalytic reduction of secondary amides to imines and aldehydes†
Abstract
Secondary amides can be reduced by silane HSiMe2Ph into imines and aldehydes by a two-stage process involving prior conversion of amides into iminoyl chlorides followed by catalytic reduction mediated by the ruthenium complex [Cp(i-Pr3P)Ru(NCCH3)2]PF6 (1). Alkyl and aryl amides bearing halogen, ketone, and ester groups were converted with moderate to good yields under mild reaction conditions to the corresponding imines and aldehydes. This procedure does not work for substrates bearing the nitro-group and fails for heteroaromatic amides. In the case of cyano substituted amides, the cyano group is reduced to imine.
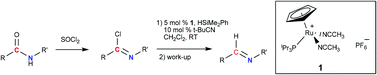

 Please wait while we load your content...
Please wait while we load your content...