Ionic liquids from cationic palladium(ii) chelate complexes: preparation, thermal properties, and crystal structures†
Abstract
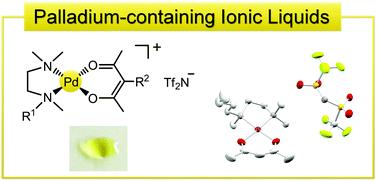
Metal-containing ionic liquids comprising cationic PdII chelate complexes and the bis(trifluoromethanesulfonyl)amide (Tf2N) anion were prepared: [Pd(acac)(Me4en)]Tf2N (1), [Pd(acac)(BuMe3en)]Tf2N (2), and [Pd(C8-acac)(Me4en)]Tf2N (3) (acac = 2,4-pentanedionate, C8-acac = 3-octyl-2,4-pentanedionate, Me4en = N,N,N′,N′-tetramethylethylenediamine, BuMe3en = N-butyl-N,N′,N′-trimethylethylenediamine). These salts were yellow solids with melting points of 85.2 °C, 71.1 °C, and 62.3 °C, respectively. During cooling from the liquid state, complex 1 exhibited crystallization, whereas 2 and 3 exhibited only glass transitions at approximately −40 °C. X-ray structure determination revealed that the cations in 1 and 3 form dimer-like arrangements and that there were no direct contacts between the charged moieties of the cations and anions in the solid state.

 Please wait while we load your content...
Please wait while we load your content...