Non-radiative relaxation of UV photoexcited phenylalanine residues: probing the role of conical intersections by chemical substitution†
Abstract
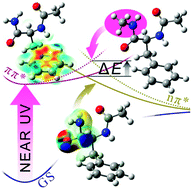
A conformation-selective photophysics study in phenylalanine model peptides, combining pump–probe gas phase experiments and excited state calculations, highlights for the first time the quenching properties of a primary amide group (through its nπ* excited state) along with the effect of vibrational energy that facilitates access to the conical intersection area.

 Please wait while we load your content...
Please wait while we load your content...