Non-selective ion channel activity of polymorphic human islet amyloid polypeptide (amylin) double channels
Abstract
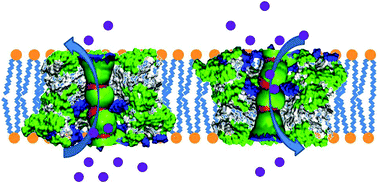
Fundamental understanding of ion channel formation by amyloid peptides, which is strongly linked to cell toxicity, is very critical for (pre)clinical treatment of neurodegenerative diseases. Here, we combine atomistic simulations and experiments to demonstrate a broad range of conformational states of hIAPP double channels in lipid membranes. All individual channels display high selectivity for Cl− ions over cations, but the co-existence of polymorphic double channels of different conformations and orientations with different populations determines the non-ionic selectivity nature of the channels, which is different from the typical amyloid-β channels that exhibit Ca2+ selective ion-permeable characteristics. This work provides a more complete physicochemical mechanism of amyloid-channel-induced toxicity.

 Please wait while we load your content...
Please wait while we load your content...