Extending the electron spin coherence time of atomic hydrogen by dynamical decoupling†
Abstract
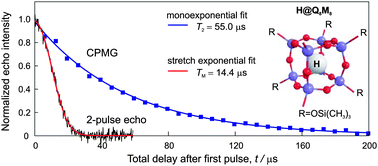
We study the electron spin decoherence of encapsulated atomic hydrogen in octasilsesquioxane cages induced by the 1H and 29Si nuclear spin bath. By applying the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence we significantly suppress the low-frequency noise due to nuclear spin flip-flops up to the point where a maximum T2 = 56 μs is observed. Moreover, dynamical decoupling with the CPMG sequence reveals the existence of two other sources of decoherence: first, a classical magnetic field noise imposed by the 1H nuclear spins of the cage organic substituents, which can be described by a virtual fluctuating magnetic field with the proton Larmor frequency, and second, decoherence due to anisotropic hyperfine coupling between the electron and the inner 29Si spins of the cage.

 Please wait while we load your content...
Please wait while we load your content...