Efficient C–C bond splitting on Pt monolayer and sub-monolayer catalysts during ethanol electro-oxidation: Pt layer strain and morphology effects†
Abstract
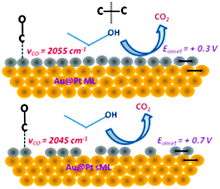
Efficient catalytic C–C bond splitting coupled with complete 12-electron oxidation of the ethanol molecule to CO2 is reported on nanoscale electrocatalysts comprised of a Pt monolayer (ML) and sub-monolayer (sML) deposited on Au nanoparticles (Au@Pt ML/sML). The Au@Pt electrocatalysts were synthesized using surface limited redox replacement (SLRR) of an underpotentially deposited (UPD) Cu monolayer in an electrochemical cell reactor. Au@Pt ML showed improved catalytic activity for ethanol oxidation reaction (EOR) and, unlike their Pt bulk and Pt sML counterparts, was able to generate CO2 at very low electrode potentials owing to efficient C–C bond splitting. To explain this, we explore the hypothesis that competing strain effects due to the Pt layer coverage/morphology (compressive) and the Pt–Au lattice mismatch (tensile) control surface chemisorption and overall activity. Control experiments on well-defined model Pt monolayer systems are carried out involving a wide array of methods such as high-energy X-ray diffraction, pair-distribution function (PDF) analysis, in situ electrochemical FTIR spectroscopy, and in situ scanning tunneling microscopy. The vibrational fingerprints of adsorbed CO provide compelling evidence on the relation between surface bond strength, layer strain and morphology, and catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...