Photo-stability of peptide-bond aggregates: N-methylformamide dimers†
Abstract
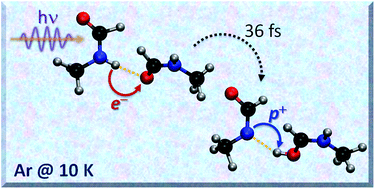
The formation of weakly-bound dimers of N-methylformamide (NMF) and the photochemistry of these dimers after irradiation at 248 nm were explored using matrix-isolation spectroscopy. Calculations were used to characterize the diverse isomers and assign their IR spectra; non-adiabatic dynamics was simulated to understand their photo-deactivation mechanism. The most stable dimers, tt-1 and tt-2, were obtained by trans–trans aggregation (N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions) and could be identified in the matrix. The main products formed after irradiation are the trans–cis dimers (tc-3 and tc-4), also stabilized by N–H⋯O
C interactions) and could be identified in the matrix. The main products formed after irradiation are the trans–cis dimers (tc-3 and tc-4), also stabilized by N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions. In contrast to the photochemistry of the monomers, no dissociative products were observed after 248 nm irradiation of the dimers. The absence of dissociative products can be explained by a proton-transfer mechanism in the excited state that is faster than the photo-dissociative mechanism. The fact that hydrogen bonding has such a significant effect on the photochemical stability of NMF has important implications to understand the stability of peptide-bonded systems to UV irradiation.
C interactions. In contrast to the photochemistry of the monomers, no dissociative products were observed after 248 nm irradiation of the dimers. The absence of dissociative products can be explained by a proton-transfer mechanism in the excited state that is faster than the photo-dissociative mechanism. The fact that hydrogen bonding has such a significant effect on the photochemical stability of NMF has important implications to understand the stability of peptide-bonded systems to UV irradiation.

 Please wait while we load your content...
Please wait while we load your content...