Controlled amino-functionalization by electrochemical reduction of bromo and nitro azobenzene layers bound to Si(111) surfaces†
Abstract
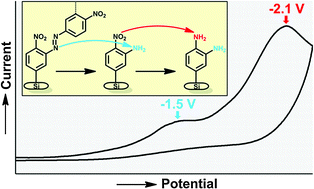
4-Nitrobenzenediazonium (4-NBD) and 4-bromobenzenediazonium (4-BBD) salts were grafted electrochemically onto H-terminated, p-doped silicon (Si) surfaces. Atomic force microscopy (AFM) and ellipsometry experiments clearly showed layer thicknesses of 2–7 nm, which indicate multilayer formation. Decreasing the diazonium salt concentration and the reaction time resulted in a smaller layer thickness, but did not prevent the formation of multilayers. It was demonstrated, mainly by X-ray photoelectron spectroscopy (XPS), that the diazonium salts not only react with the H-terminated Si surface, but also with electrografted phenyl groups via azo-bond formation. These azo bonds can be electrochemically reduced at Ered = −1.5 V, leading to the corresponding amino groups. This reduction resulted in a modest decrease in layer thickness, and did not yield monolayers. This indicates that other coupling reactions, notably a biphenyl coupling, induced by electrochemically produced phenyl radicals, take place as well. In addition to the azo functionalities, the nitro functionalities in electrografted layers of 4-NBD were independently reduced to amino functionalities at a lower potential (Ered = −2.1 V). The presence of amino functionalities on fully reduced layers, both from 4-NBD- and 4-BBD-modified Si, was shown by the presence of fluorine after reaction with trifluoroacetic anhydride (TFAA). This study shows that the electrochemical reduction of azo bonds generates amino functionalities on layers produced by electrografting of aryldiazonium derivatives. In this way multifunctional layers can be formed by employing functional aryldiazonium salts, which is believed to be very practical in the fabrication of sensor platforms, including those made of multi-array silicon nanowires.

 Please wait while we load your content...
Please wait while we load your content...