Nickel–silver alloy electrocatalysts for hydrogen evolution and oxidation in an alkaline electrolyte†
Abstract
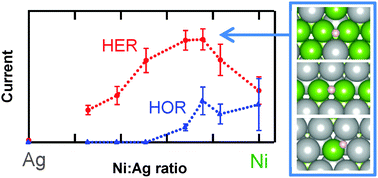
The development of improved catalysts for the hydrogen evolution reaction (HER) and hydrogen oxidation reaction (HOR) in basic electrolytes remains a major technical obstacle to improved fuel cells, water electrolyzers, and other devices for electrochemical energy storage and conversion. Based on the free energy of adsorbed hydrogen intermediates, theory predicts that alloys of nickel and silver are active for these reactions. In this work, we synthesize binary nickel–silver bulk alloys across a range of compositions and show that nickel–silver alloys are indeed more active than pure nickel for hydrogen evolution and, possibly, hydrogen oxidation. To overcome the mutual insolubility of silver and nickel, we employ electron-beam physical vapor codeposition, a low-temperature synthetic route to metastable alloys. This method also produces flat and uniform films that facilitate the measurement of intrinsic catalytic activity with minimal variations in the surface area, ohmic contact, and pore transport. Rotating-disk-electrode measurements demonstrate that the hydrogen evolution activity per geometric area of the most active catalyst in this study, Ni0.75Ag0.25, is approximately twice that of pure nickel and has comparable stability and hydrogen oxidation activity. Our experimental results are supported by density functional theory calculations, which show that bulk alloying of Ni and Ag creates a variety of adsorption sites, some of which have near-optimal hydrogen binding energy.

 Please wait while we load your content...
Please wait while we load your content...